Chapter 46 Species Found on the Galapagos Islands
46.1 A Little Background
The gala data describe describe features of the 30 Galapagos Islands.
The Galapagos Islands are found about 900 km west of South America: specifically the continental part of Ecuador. The Islands form a province of Ecuador and serve as a national park and marine reserve. They are noted for their vast numbers of unique (or endemic) species and were studied by Charles Darwin during the voyage of the Beagle. I didn’t know most of this, but it’s on Wikipedia, so I’ll assume it’s all true until someone sets me straight.
galapic.jpg
46.1.1 Sources
The data were initially presented by Johnson M and Raven P (1973) Species number and endemism: the Galapagos Archipelago revisited. Science 179: 893-895 and also appear in several regression texts, including my source: Faraway (2015). Note that Faraway filled in some missing data to simplify things a bit. A similar version of the data is available as part of the faraway library in R, but I encourage you to use the version I supply on our web site.
46.1.2 Variables in the gala data frame
- id = island identification code
- island = island name
- species = our outcome, the number of species found on the island
- area = the area of the island, in square kilometers
- elevation = the highest elevation of the island, in meters
- nearest = the distance from the nearest island, in kilometers
- scruz = the distance from Santa Cruz Island, in kilometers. Santa Cruz is the home to the largest human population in the Islands, and to the town of Puerto Ayora.
- adjacent = the area of the adjacent island, in square kilometers
gala# A tibble: 30 x 8
id island species area elevation nearest scruz adjacent
<int> <fctr> <int> <dbl> <int> <dbl> <dbl> <dbl>
1 1 Baltra 58 25.09 346 0.6 0.6 1.84
2 2 Bartolome 31 1.24 109 0.6 26.3 572.33
3 3 Caldwell 3 0.21 114 2.8 58.7 0.78
4 4 Champion 25 0.10 46 1.9 47.4 0.18
5 5 Coamano 2 0.05 77 1.9 1.9 903.82
6 6 Daphne.Major 18 0.34 119 8.0 8.0 1.84
7 7 Daphne.Minor 24 0.08 93 6.0 12.0 0.34
8 8 Darwin 10 2.33 168 34.1 290.2 2.85
9 9 Eden 8 0.03 71 0.4 0.4 17.95
10 10 Enderby 2 0.18 112 2.6 50.2 0.10
# ... with 20 more rowsHmisc::describe(gala) # check for missing and inexplicable valuesgala
8 Variables 30 Observations
---------------------------------------------------------------------------
id
n missing distinct Info Mean Gmd .05 .10
30 0 30 1 15.5 10.33 2.45 3.90
.25 .50 .75 .90 .95
8.25 15.50 22.75 27.10 28.55
lowest : 1 2 3 4 5, highest: 26 27 28 29 30
---------------------------------------------------------------------------
island
n missing distinct
30 0 30
lowest : Baltra Bartolome Caldwell Champion Coamano
highest: SantaFe SantaMaria Seymour Tortuga Wolf
---------------------------------------------------------------------------
species
n missing distinct Info Mean Gmd .05 .10
30 0 27 0.999 85.23 109.5 2.0 2.9
.25 .50 .75 .90 .95
13.0 42.0 96.0 280.5 319.1
lowest : 2 3 5 8 10, highest: 237 280 285 347 444
---------------------------------------------------------------------------
area
n missing distinct Info Mean Gmd .05 .10
30 0 29 1 261.7 478.6 0.0390 0.0770
.25 .50 .75 .90 .95
0.2575 2.5900 59.2375 578.5460 782.6215
Value 0 20 30 60 130 170 550 570 630 900
Frequency 17 3 1 2 1 1 1 1 1 1
Proportion 0.567 0.100 0.033 0.067 0.033 0.033 0.033 0.033 0.033 0.033
Value 4670
Frequency 1
Proportion 0.033
---------------------------------------------------------------------------
elevation
n missing distinct Info Mean Gmd .05 .10
30 0 30 1 368 411.1 47.35 68.80
.25 .50 .75 .90 .95
97.75 192.00 435.25 868.20 1229.40
lowest : 25 46 49 71 76, highest: 777 864 906 1494 1707
---------------------------------------------------------------------------
nearest
n missing distinct Info Mean Gmd .05 .10
30 0 22 0.997 10.06 13.73 0.445 0.590
.25 .50 .75 .90 .95
0.800 3.050 10.025 34.100 40.205
lowest : 0.2 0.4 0.5 0.6 0.7, highest: 16.5 29.1 34.1 45.2 47.4
---------------------------------------------------------------------------
scruz
n missing distinct Info Mean Gmd .05 .10
30 0 29 1 56.98 65.17 0.49 0.60
.25 .50 .75 .90 .95
11.02 46.65 81.08 97.73 193.90
lowest : 0.0 0.4 0.6 1.9 8.0, highest: 93.1 95.3 119.6 254.7 290.2
---------------------------------------------------------------------------
adjacent
n missing distinct Info Mean Gmd .05 .10
30 0 21 0.998 261.1 477.8 0.10 0.10
.25 .50 .75 .90 .95
0.52 2.59 59.24 578.55 782.62
Value 0 20 30 60 130 570 630 900 4670
Frequency 17 2 2 2 2 2 1 1 1
Proportion 0.567 0.067 0.067 0.067 0.067 0.067 0.033 0.033 0.033
---------------------------------------------------------------------------46.2 DTDP: A Scatterplot Matrix
After missingness and range checks, the first step in any data analysis problem is to draw the picture. The most useful picture for me in thinking about a regression problem with a reasonably small number of predictors is a scatterplot matrix.
Our outcome, that we are predicting here is the number of species.
We’ll use five predictors:
areaelevationnearestscruzandadjacent.
pairs (~ species + area + elevation + nearest + scruz + adjacent,
data=gala, main="Galapagos Islands Matrix",
upper.panel = panel.smooth,
diag.panel = panel.hist,
lower.panel = panel.cor)
46.2.1 Questions about the Scatterplot Matrix
- What are we looking for in the scatterplots in the top row?
- What can we learn from the Pearson correlations in the left column?
- How do the histograms help increase our understanding of the data?
- What about the scatterplots that are not in the top row?
- What can we learn from the Pearson correlations that compare predictors?
46.3 Fitting A “Kitchen Sink” Linear Regression model
Next, we’ll fit a multiple linear regression model to predict the number of species based on the five predictors included in the gala data frame (and scatterplot matrix above.) We use the lm command to fit the linear model, and use what is called Wilkinson-Rogers notation to specify the model.
model1 <- lm(species ~ area + elevation + nearest + scruz +
adjacent, data=gala)
summary(model1)
Call:
lm(formula = species ~ area + elevation + nearest + scruz + adjacent,
data = gala)
Residuals:
Min 1Q Median 3Q Max
-111.68 -34.90 -7.86 33.46 182.58
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 7.06822 19.15420 0.37 0.7154
area -0.02394 0.02242 -1.07 0.2963
elevation 0.31946 0.05366 5.95 3.8e-06 ***
nearest 0.00914 1.05414 0.01 0.9932
scruz -0.24052 0.21540 -1.12 0.2752
adjacent -0.07480 0.01770 -4.23 0.0003 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 61 on 24 degrees of freedom
Multiple R-squared: 0.766, Adjusted R-squared: 0.717
F-statistic: 15.7 on 5 and 24 DF, p-value: 6.84e-0746.3.1 Questions about the Kitchen Sink Model Summaries
What conclusions can we draw from the summary output for this model? Specifically …
- What is being predicted? What is the prediction equation?
- How do we interpret the
elevationestimate of 0.32? - How do we interpret the
areaestimate of -0.02? - How do we interpret the intercept estimate of 7.07?
- Overall, does the model add statistically significant predictive value over the simplest possible model, using the intercept term alone?
- What proportion of the variation in
speciescounts does this model account for? - What does the residual standard error mean in this context?
- What can we learn from the standard errors in the coefficient output?
- What can we learn from the
tvalues andPr(>|t|)values in the coefficient output? - How should we interpret the meaning of the
Adjusted R-squaredvalue?
46.4 Finding Confidence Intervals for our Coefficient Estimates
confint(model1, level = 0.95) 2.5 % 97.5 %
(Intercept) -32.4641 46.6005
area -0.0702 0.0223
elevation 0.2087 0.4302
nearest -2.1665 2.1848
scruz -0.6851 0.2040
adjacent -0.1113 -0.038346.4.1 Questions about the Confidence Intervals
- What can we learn from the provided confidence interval for
elevation? - How do the confidence interval results here compare to the t tests in the
summaryoutput?
46.5 Measuring Collinearity - the Variance Inflation Factor
The variance inflation factor (abbreviated VIF) can be used to quantify the impact of multicollinearity in a linear regression model.
The VIF is sometimes interpreted by taking its square root, and then interpreting the result as telling you how much larger the standard error for that coefficient is, as compared to what it would be if that variable were uncorrelated with the other predictors.
In R, the vif function from the car library, when applied to a linear regression model, specifies the variance inflation factors for each of the model’s coefficients, as follows.
vif(model1) area elevation nearest scruz adjacent
2.93 3.99 1.77 1.68 1.83 So, for instance, the VIF of 3.99 for elevation implies that the standard error of the elevation coefficient is approximately 2 times larger than it would be if elevation was uncorrelated with the other predictors.
I will look closely at any VIF value that is greater than 5, although some people use a cutoff of 10.
- Another collinearity measure called tolerance is simply 1/
VIF. - For example, the tolerance for
elevationwould be 0.25, and the cutoff for a potentially problematic tolerance is either 0.2 or lower, or 0.1 or lower.
To calculate the VIF for a predictor \(x_1\), use all of the other predictors to predict \(x_1\) and find the multiple R2 value.
VIFfor \(x_1\) = 1 / (1 - \(R^2_{x_1 | others}\)), andtolerance= (1 - \(R^2_{x_1 | others}\)).
46.6 Global (F) Testing of Overall Significance
Our Galapagos Islands species count regression model (called model1) predicts the count of an island’s species using area, elevation, nearest, scruz and adjacent.
nullmodel <- lm(species ~ 1, data=gala)
summary(nullmodel)
Call:
lm(formula = species ~ 1, data = gala)
Residuals:
Min 1Q Median 3Q Max
-83.2 -72.2 -43.2 10.8 358.8
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 85.2 20.9 4.07 0.00033 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 115 on 29 degrees of freedomanova(model1, nullmodel)Analysis of Variance Table
Model 1: species ~ area + elevation + nearest + scruz + adjacent
Model 2: species ~ 1
Res.Df RSS Df Sum of Sq F Pr(>F)
1 24 89231
2 29 381081 -5 -291850 15.7 6.8e-07 ***
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 146.6.1 Questions about the Global Test via ANOVA
- How do we interpret the null model fit above?
- What are the hypotheses being tested by this ANOVA output?
- What conclusions can we draw from the ANOVA output presented here?
- Where do we find information regarding the result for the previous question in the summary output for the linear model?
- How would we set up an ANOVA model to test whether the “kitchen sink” model’s predictive value would be significantly impacted by removing the
adjacentpredictor from the model? - Where do we find information regarding these result for the previous question in the
summaryoutput for the linear model? - How would we set an ANOVA model to test whether a model with
areaonly would be a significant improvement over the null model?
46.7 Sequential Testing in a Regression Model with ANOVA
anova(model1)Analysis of Variance Table
Response: species
Df Sum Sq Mean Sq F value Pr(>F)
area 1 145470 145470 39.13 1.8e-06 ***
elevation 1 65664 65664 17.66 0.00032 ***
nearest 1 29 29 0.01 0.93007
scruz 1 14280 14280 3.84 0.06173 .
adjacent 1 66406 66406 17.86 0.00030 ***
Residuals 24 89231 3718
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 146.7.1 Questions about Sequential Testing and ANOVA
- What conclusions can we draw from the
arearow in the output above? - What conclusions can we draw from the
elevationrow? - Does
nearestadd statistically significant predictive value to the model includingareaandelevation, but none of the other predictors? - Does
adjacentadd significant predictive value as last predictor into the model? - Where else in the regression output can we find the answer to the previous question?
- How does the mean square of the residuals (3718) relate to the residual standard error?
- What percentage of the variation in the species counts is accounted for by area alone?
- What percentage of the variation explained by the kitchen sink model would also be accounted for in a two-predictor regression model including
areaandelevationalone? - How could we use the original linear model output to whether a model using the four predictors that appear most promising here would be statistically significantly worse than the full model with all five predictors?
- What does the following output do differently than the output above, and why is that potentially useful here? Why is the p value for
scruzso different?
anova(lm(species ~ area + elevation + adjacent + scruz + nearest, data=gala))Analysis of Variance Table
Response: species
Df Sum Sq Mean Sq F value Pr(>F)
area 1 145470 145470 39.13 1.8e-06 ***
elevation 1 65664 65664 17.66 0.00032 ***
adjacent 1 73171 73171 19.68 0.00017 ***
scruz 1 7544 7544 2.03 0.16719
nearest 1 0 0 0.00 0.99315
Residuals 24 89231 3718
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1- Consider the ANOVA below, run on a new model with
elevationafteradjacent. What happens? Why?
anova(lm(species ~ area + adjacent + elevation + scruz + nearest, data=gala))Analysis of Variance Table
Response: species
Df Sum Sq Mean Sq F value Pr(>F)
area 1 145470 145470 39.13 1.8e-06 ***
adjacent 1 2850 2850 0.77 0.39
elevation 1 135985 135985 36.58 3.0e-06 ***
scruz 1 7544 7544 2.03 0.17
nearest 1 0 0 0.00 0.99
Residuals 24 89231 3718
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 146.8 An ANOVA table for the Model as a Whole
It’s probably also worthwhile to compute a completed ANOVA table for the model as a whole. All elements are in the ANOVA tables above, or the model summary.
| Group | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| Regression | 5 | 291849 | 58369.8 | 15.7 | 6.838e-07 |
| Residuals | 24 | 89231 | 3718.0 | ||
| Total | 29 | 381080 |
- How did I determine the Mean Square for the Regression model?
- What conclusions can we draw from this ANOVA table?
46.9 Assumption Checking for our Galápagos Islands models
Remember that the key assumptions of multiple linear regression are:
- [Linearity] We have also assumed that the structural part of the model is correctly specified (we’ve included all the predictors that need to be in the model, and expressed them in the most appropriate manner, and we’ve left out any predictors that don’t need to be in the model.)
- [Normality] The regression makes errors that come from a Normal distribution
- [Homoscedasticity = Constant Variance] The regression makes errors that come from a distribution with constant variance at all predictor levels.
- [Independence] The regression errors are independent of each other.
In addition, we need to realize that sometimes a few observations may be particularly problematic. For instance:
- An observation may simply not fit the model well (i.e. it creates a large residual)
- An observation may have high leverage over the fit of the model (this happens with observations that have an unusual combination of predictor values, in particular)
- An observation may actually have high influence on the model (in the sense that whether they are included or excluded has a large impact on the model’s fit, and the value of its parameter estimates.)
- Or any combination of high residual, leverage and influence may occur.
So it is important to check the assumptions that we can with the data we have. Our most important tools are plots and other summaries of residuals, and what are called influence statistics.
46.10 My First Plot: Studentized Residuals vs. Fitted Values
The first diagnostic plot I usually draw for a multiple regression is a scatterplot of the model’s studentized residuals (on the vertical axis) vs. the model’s fitted values (on the horizontal.) This plot can be used to assess potential non-linearity, non-constant variance, and non-Normality in the residuals.
gala$stures <- rstudent(model1); gala$fits <- fitted(model1)
ggplot(gala, aes(x = fits, y = stures)) +
theme_bw() + geom_point(size = 3, shape = 1) +
geom_smooth(col = "blue", se = FALSE, weight = 0.5) +
geom_hline(aes(yintercept = 0), linetype = "dashed") +
labs(x = "Fitted Values of Species",
y = "Studentized Residuals",
title = "gala data: Model 1\nStudentized Residuals vs. Fitted Values")`geom_smooth()` using method = 'loess'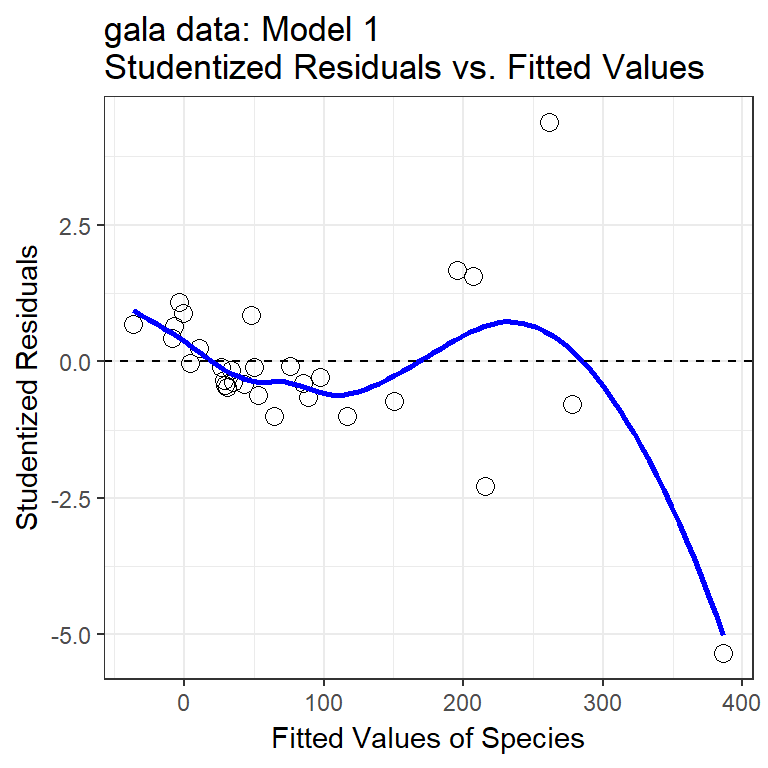
46.10.1 Questions about Studentized Residuals vs. Fitted Values
- Consider the point at bottom right. What can you infer about this observation?
- Why did I include the dotted horizontal line at Studentized Residual = 0?
- What is the purpose of the thin blue line?
- What does this plot suggest about the potential for outliers in the residuals?
46.11 Automatic Regression Diagnostics for Model 1
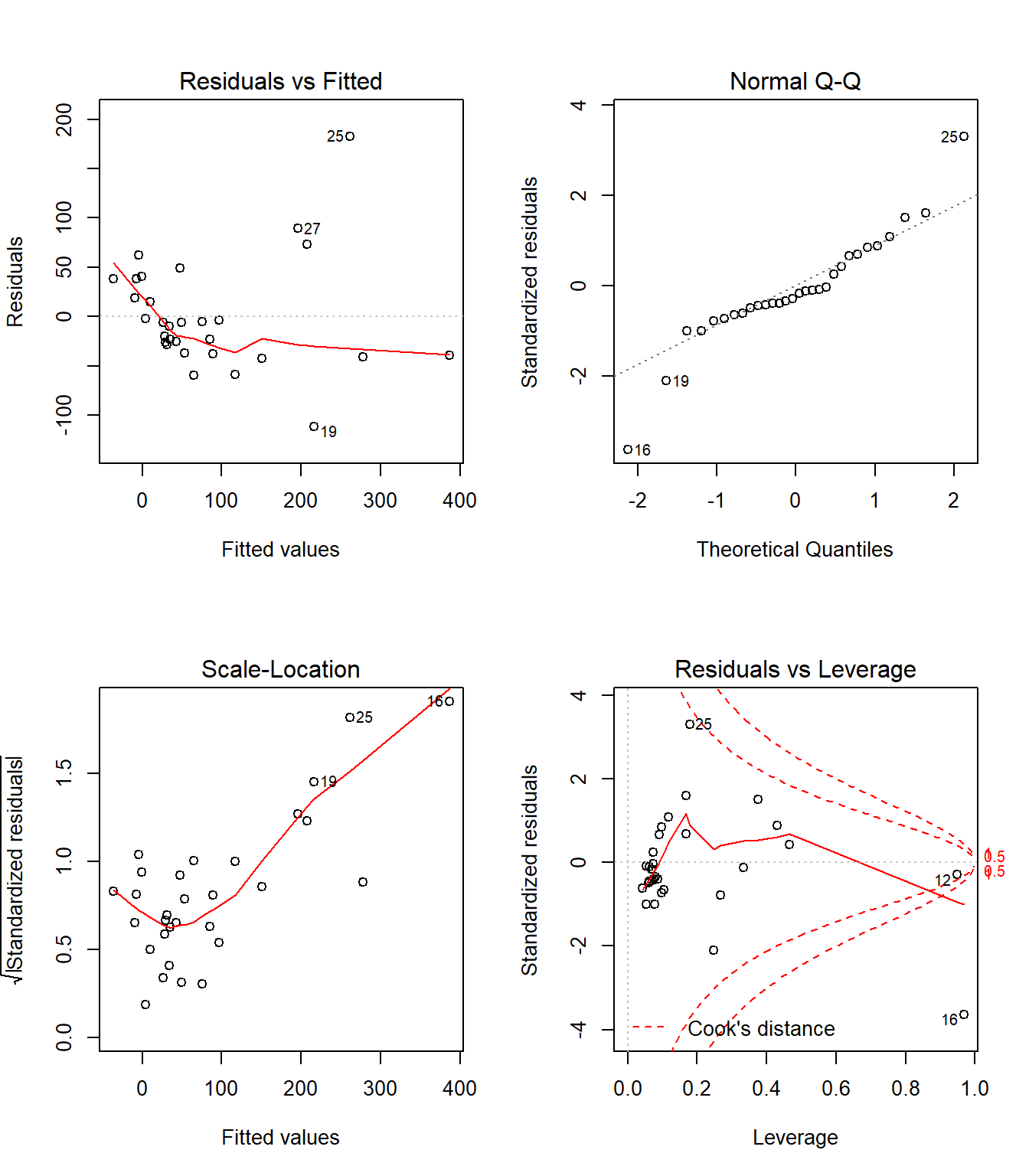
46.12 Model 1: Diagnostic Plot 1
As we’ve seen, the first of R’s automated diagnostic plots for a linear model is a plot of the residuals vs. the fitted values.
plot(model1, which=1)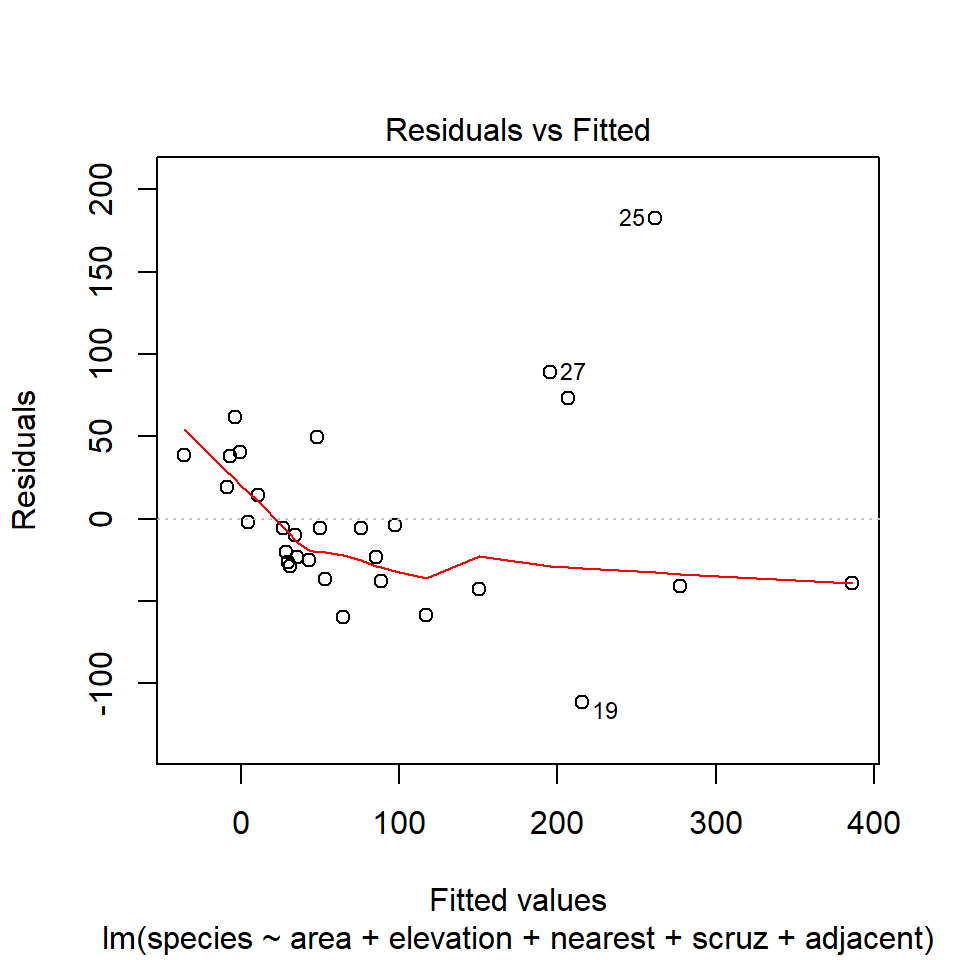
46.12.1 Questions about Diagnostic Plot 1: Residuals vs. Fitted Values
- What type of regression residuals is R plotting here?
- Which points are identified by numbers here?
- Why did R include the gray dotted line at Residual = 0?
- What is the purpose of the thin red line?
- What can you tell about the potential for outliers in the model 1 residuals from the plot?
- What are we looking for in this plot that would let us conclude there were no important assumption violations implied by it? Which assumptions can we assess with it?
- What would we do if we saw a violation of assumptions in this plot?
- What are the key differences between this plot and the one I showed earlier?
46.13 Diagnostic Plot 2: Assessing Normality
The second diagnostic plot prepared by R for any linear model using the plot command is a Normal Q-Q plot of the standardized residuals from the model.
plot(model1, which=2)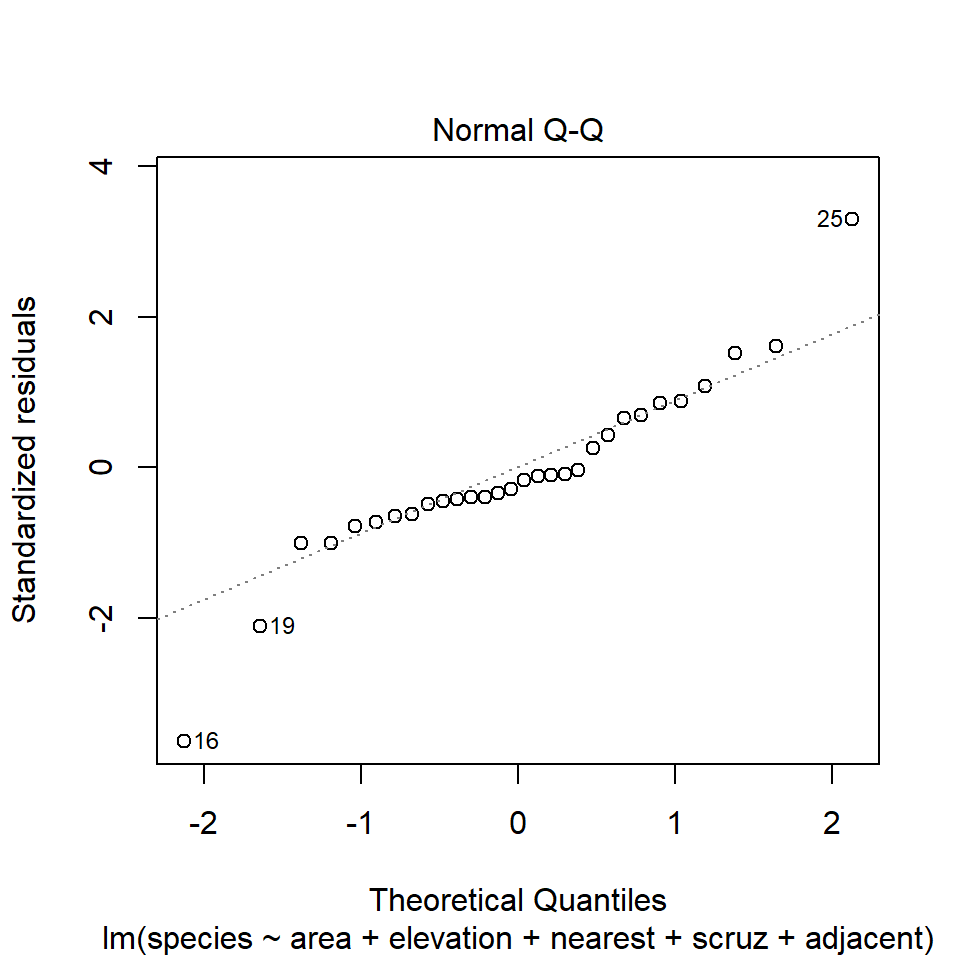
46.13.1 Questions about Diagnostic Plot 2: Normal Plot of Standardized Residuals
- Which points are being identified here by number?
- Which assumption(s) of multiple regression does this plot help us check?
- What are we looking for in this plot that would let us conclude there were no important assumption violations implied by it?
- What would we do if we saw a violation of assumptions in this plot?
We could also look at studentized residuals, or we could apply a more complete set of plots and other assessments of normality. Usually, I don’t.
46.14 Diagnostic Plot 3: Assessing Constant Variance
The third diagnostic plot prepared by R for any linear model using the plot command shows the square root of the model’s standardized residuals vs. its fitted values. R calls this a scale-location plot.
plot(model1, which=3)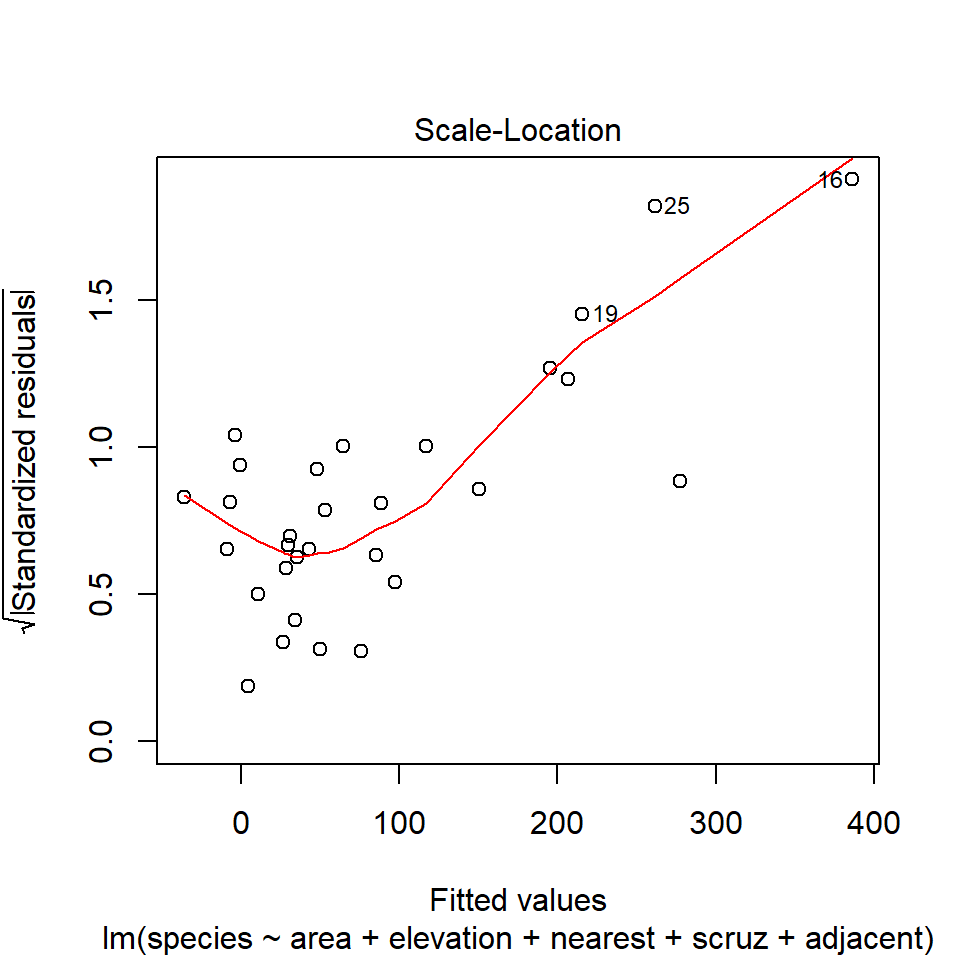
46.14.1 Questions about Diagnostic Plot 3: Scale-Location Plot
- Which points are being identified here by number?
- Which assumption(s) of multiple regression does this plot help us check?
- What is the role of the thin red line in this plot?
- What are we looking for in this plot that would let us conclude there were no important assumption violations implied by it?
- What would we do if we saw a violation of assumptions in this plot?
46.15 Obtaining Fitted Values and Residuals from a Model
Remember that we can use the fitted function applied to a model to find the predictions made by the regression model for each of the observations used to create the model.
round(fitted(model1),2) 1 2 3 4 5 6 7 8 9 10
116.73 -7.27 29.33 10.36 -36.38 43.09 33.92 -9.02 28.31 30.79
11 12 13 14 15 16 17 18 19 20
47.66 96.99 -4.03 64.63 -0.50 386.40 88.69 4.04 215.68 150.48
21 22 23 24 25 26 27 28 29 30
35.08 75.55 206.95 277.68 261.42 85.38 195.62 49.81 52.94 26.70 gala[1,]# A tibble: 1 x 10
id island species area elevation nearest scruz adjacent stures fits
<int> <fctr> <int> <dbl> <int> <dbl> <dbl> <dbl> <dbl> <dbl>
1 1 Baltra 58 25.1 346 0.6 0.6 1.84 -1 11746.15.1 Questions about Fitted Values
Verify that the first fitted value [116.73] is in fact what you get for Baltra (observation 1) when you apply the regression equation:
species = 7.07 - 0.02 area + 0.32 elevation + 0.009 nearest - 0.24 scruz - 0.07 adjacent
We can compare these predictions to the actual observed counts of the number of species on each island. Subtracting the fitted values from the observed values gives us the residuals, as does the resid function.
round(resid(model1),2) 1 2 3 4 5 6 7 8 9
-58.73 38.27 -26.33 14.64 38.38 -25.09 -9.92 19.02 -20.31
10 11 12 13 14 15 16 17 18
-28.79 49.34 -3.99 62.03 -59.63 40.50 -39.40 -37.69 -2.04
19 20 21 22 23 24 25 26 27
-111.68 -42.48 -23.08 -5.55 73.05 -40.68 182.58 -23.38 89.38
28 29 30
-5.81 -36.94 -5.70 46.15.2 Questions about Residuals
- What does a positive residual indicate?
- What does a negative residual indicate?
- The standard deviation of the full set of 30 residuals turns out to be 55.47. How does this compare to the residual standard error?
- The command below identifies Santa Cruz. What does it indicate about Santa Cruz, specifically?
gala$island[which.max(resid(model1))][1] SantaCruz
30 Levels: Baltra Bartolome Caldwell Champion Coamano ... Wolf- From the results below, what is the
model1residual for Santa Cruz? What does this imply about thespeciesprediction made by Model 1 for Santa Cruz?
which.max(resid(model1))25
25 round(resid(model1),2) 1 2 3 4 5 6 7 8 9
-58.73 38.27 -26.33 14.64 38.38 -25.09 -9.92 19.02 -20.31
10 11 12 13 14 15 16 17 18
-28.79 49.34 -3.99 62.03 -59.63 40.50 -39.40 -37.69 -2.04
19 20 21 22 23 24 25 26 27
-111.68 -42.48 -23.08 -5.55 73.05 -40.68 182.58 -23.38 89.38
28 29 30
-5.81 -36.94 -5.70 gala[which.max(resid(model1)),]# A tibble: 1 x 10
id island species area elevation nearest scruz adjacent stures
<int> <fctr> <int> <dbl> <int> <dbl> <dbl> <dbl> <dbl>
1 25 SantaCruz 444 904 864 0.6 0 0.52 4.38
# ... with 1 more variables: fits <dbl>46.16 Relationship between Fitted and Observed Values
We’ve already seen that the fitted command can produce predicted values for each observations used to develop a regression model, and that the resid command can produce the residuals (observed - predicted) for those same observations. Returning to our original model1, let’s compare the fitted values (stored earlier in fits) to the observed values.
ggplot(gala, aes(x = species, y = fits)) +
geom_point(size = 3, shape = 1) + theme_bw() +
geom_abline(intercept = 0, slope = 1, col = "purple", linetype = "dashed") +
labs(x = "Observed Species Count", y = "Model Predicted Species")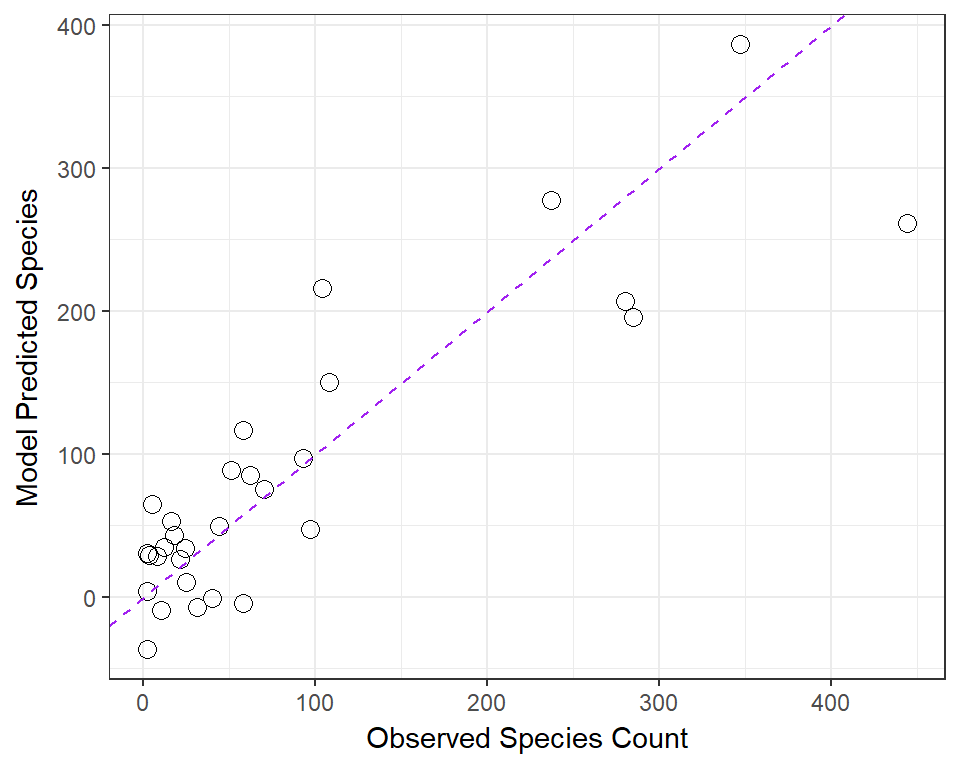
46.16.1 Questions about Fitted and Observed Values
- Why did I draw the dotted purple line with y-intercept 0 and slope 1? Why is that particular line of interest?
- If a point on this plot is in the top left here, above the dotted line, what does that mean?
- If a point is below the dotted line here, what does that mean?
- How does this plot display the size of an observation’s residual?
46.17 Standardizing Residuals
We’ve already seen that the raw residuals from a regression model can be obtained using the resid function. Residuals are defined to have mean 0. This is one of the requirements of the least squares procedure for estimating a linear model, and their true standard deviation is effectively estimated using the residual standard error.
There are two additional types of residuals for us to be aware of: standardized residuals, and studentized (sometimes called externally standardized, or jackknife) residuals. Each approach standardizes the residuals by dividing them by a standard deviation estimate, so the resulting residuals should have mean 0 and standard deviation 1 if assumptions hold.
- Standardized residuals are the original (raw) residuals, scaled by a standard deviation estimate developed using the entire data set.
- Studentized residuals are the original (raw) residuals, scaled by a standard deviation estimate developed using the entire data set EXCEPT for this particular observation.
The rstandard function, when applied to a linear regression model, will generate the standardized residuals, while rstudent generates the model’s studentized residuals.
`geom_smooth()` using method = 'loess'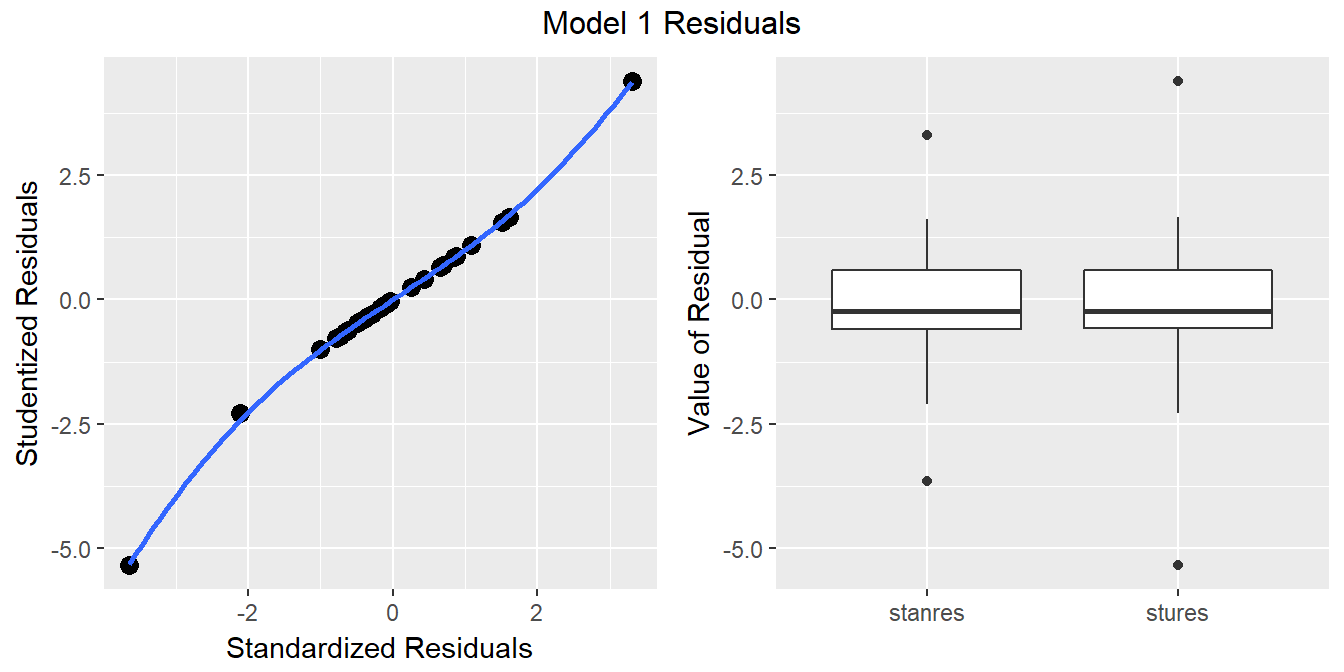
46.17.1 Questions about Standardized and Studentized Residuals
- From the plots above, what conclusions can you draw about the two methods of standardizing residuals as they apply in the case of our model1?
46.18 Three Types of Residuals
gala.res <- data.frame(raw.residuals = resid(model1),
standardized = rstandard(model1),
studentized = rstudent(model1)) %>% tbl_df
gala.res_long <- gather(gala.res, key = "type", value = "res")
ggplot(gala.res_long, aes(x = type, y = res)) +
geom_boxplot() +
coord_flip() +
labs(x = "", y = "Residual", title = "3 Types of Residuals for Model 1")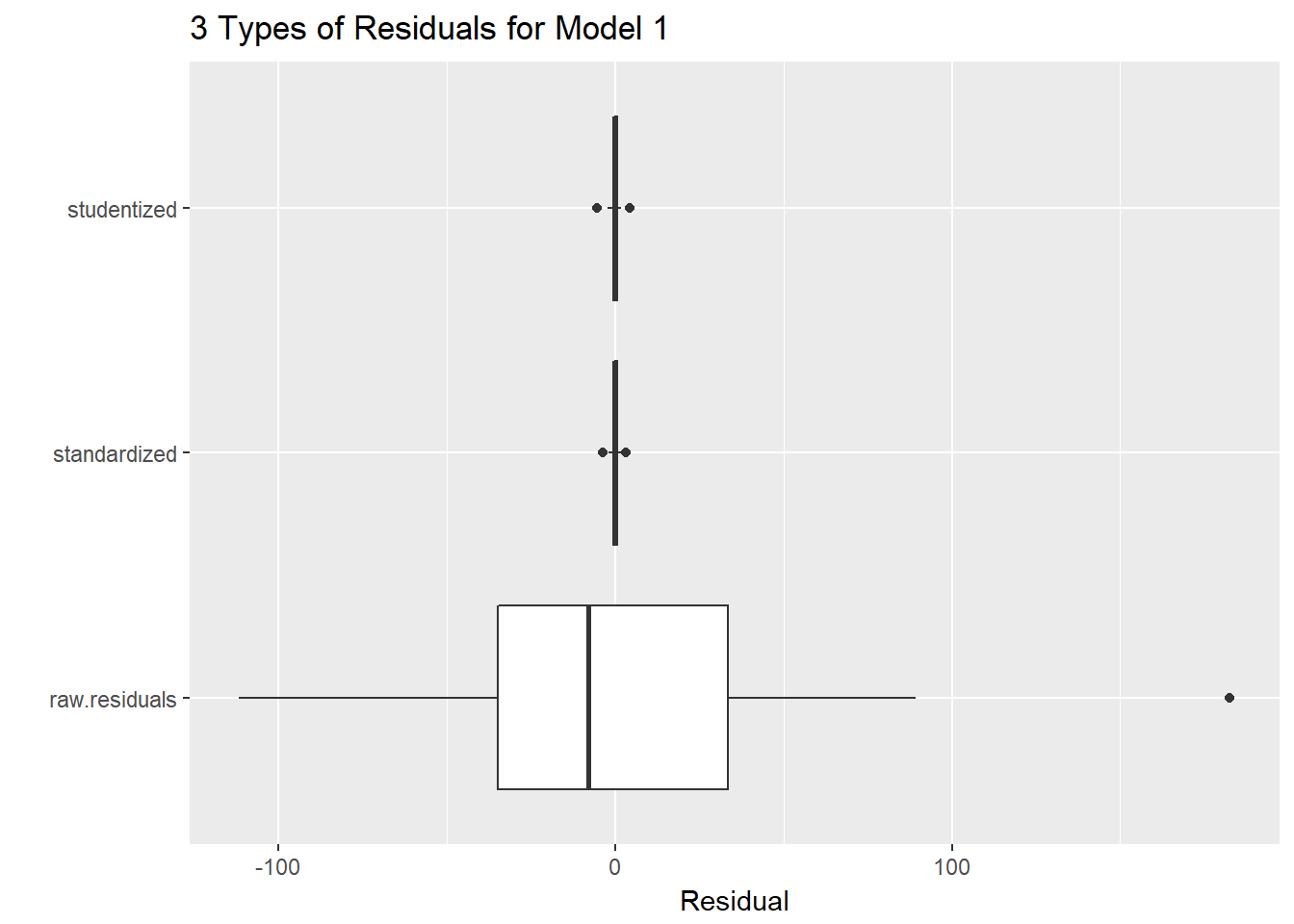
46.18.1 Questions about Three Types of Residuals
- Consider the three types of residuals, shown above. Can you specify a reason why looking at the raw residuals might be helpful in this case?
- Why might (either of the two approaches to) standardizing be useful?
Does there seem to be a substantial problem with Normality in the residuals?
How about the Normality of the studentized residuals? Which seems clearer?
eda.1sam(dataframe = gala.res, variable = gala.res$raw.residuals,
x.title = "Raw Residuals", ov.title = "Model 1: Raw Residuals")Loading required package: gridExtra
Attaching package: 'gridExtra'The following object is masked from 'package:Hmisc':
combineThe following object is masked from 'package:dplyr':
combine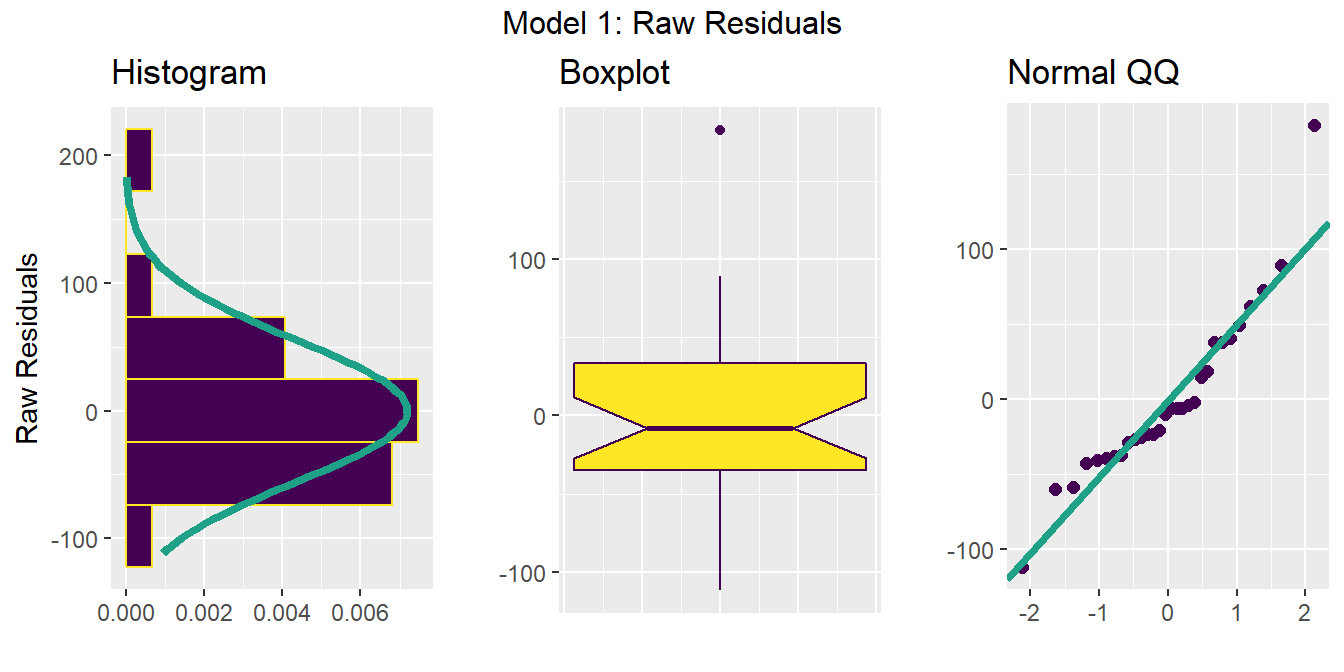
eda.1sam(dataframe = gala.res, variable = gala.res$studentized,
x.title = "Studentized Residuals",
ov.title = "Model 1: Studentized Residuals")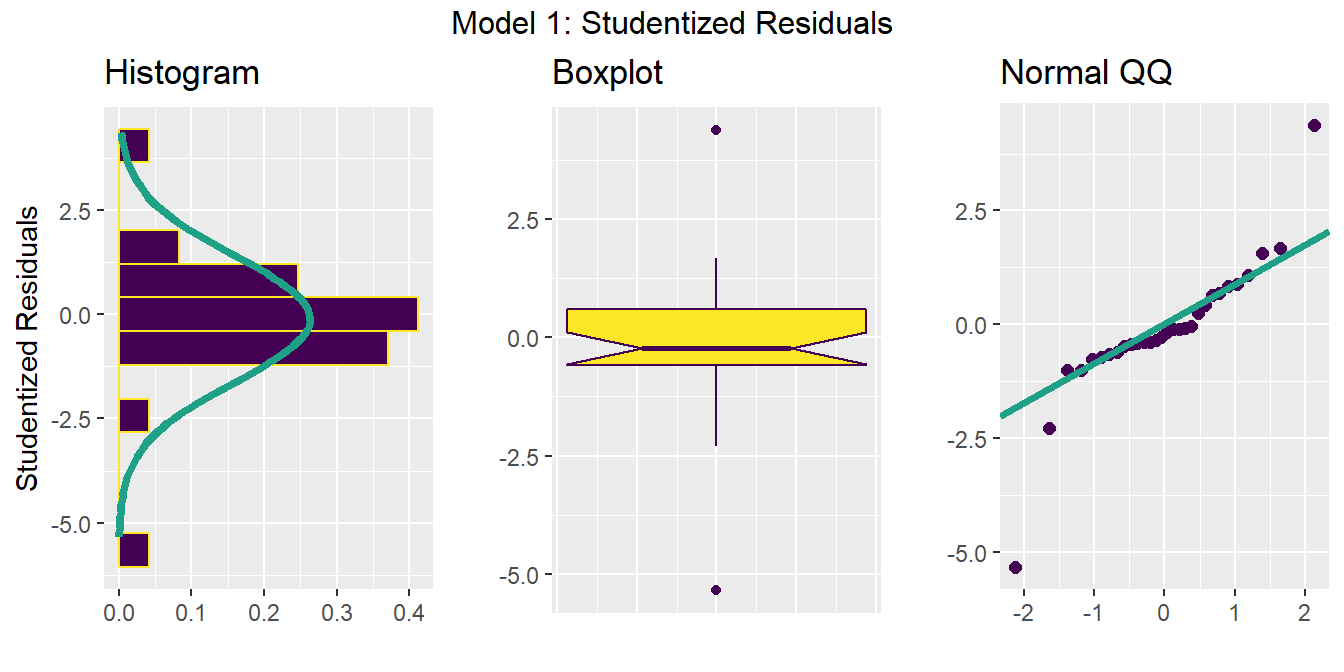
References
Faraway, Julian J. 2015. Linear Models with R. Second. Boca Raton, FL: CRC Press.